Description
BENZAC MR AC
GALDERMA
Gel
Generic name: Benzoyl peroxide.
Pharmaceutical Form and Formulation: Gel. Each 100 g contains: benzoyl peroxide. 2.5 g, 5 g and 10 g. Excipient cbp 100 g.
Therapeutic indications: BENZAC MR AC is indicated as an aid in the treatment of acne vulgaris. BENZAC MR AC contains: micronized benzoyl peroxide, which provides penetration into the pilosebaceous follicles, eliminating Propionibacterium acnes, also provides keratolytic and secant action. It is a water-based gel that reduces potential irritation caused by a common benzoyl peroxide therapy. Thanks to its formulation, it is compatible with other topical acne treatments.
Pharmacokinetics and pharmacodynamics: The mechanism of action of benzoyl peroxide is not fully cleared, but its antibacterial action against Propionibacterium acnesis its main mode of action. In addition, patients treated with benzoyl peroxide show a reduction in lipids and fatty acids and moderate desquamation (drying and exfoliating action) resulting in decreased comedones and other acne lesions. Little is known about percutaneous penetration, metabolism, and excretion of benzoyl peroxide, even though it is known that benzoyl peroxide absorbed percutaneously is metabolized by cysteine to benzoic acid and oxygen free radicals which are the ones who finally act at the level of the bacterial cell wall, being responsible for the antibacterial activity. Benzoic acid is excreted as benzoate in the urine. There is no evidence of systemic toxicity caused by the use of benzoyl peroxide in humans.
Contraindications: Hypersensitivity to the component of the formula.
General Precautions: BENZAC MR AC is for topical use only. If the medication is applied excessively, it will not get better results or faster, on the contrary, it can cause irritation, that cease when adjusting the dose.
Restrictions on use during pregnancy and lactation: There are no data on the date of adverse effects during pregnancy or lactation. However the use of the product should be assessed by the physician according to the benefit.
Secondary and adverse reactions:With the use of benzoyl peroxide, rare cases of allergic dermatitis and dryness have been reported. Erythema, pruritus, and burning sensation may also occur at the site of application during the first days of treatment, which disappear spontaneously.
Drug and other interactions: None known to date.
Changes in laboratory test results: Not reported to date.
Precautions regarding carcinogenesis, mutagenesis, teratogenicity and fertility effects : There is no clinical evidence of carcinogenic effects from the use of BENZAC MR AC. So well, benzoyl peroxide has not been shown to be mutagenic (Ames’ test) and no data showing changes in fertility have been published.
Dosage and route of administration: Route of administration: cutaneous. Wash with a cleaner or with mild soap and water, dry thoroughly and apply 1 or 2 times a day covering the affected area.
Manifestations and handling of overdose or accidental ingestion: By the characteristics of the product not present In case of accidental intake is recommended general measures of support.
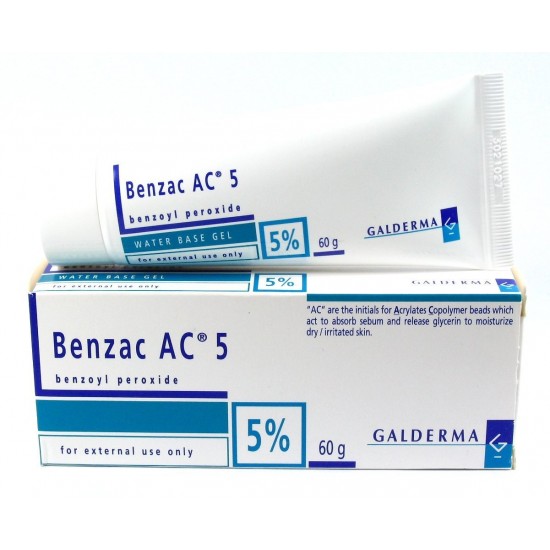
Presentation (s): Box with tube with 60 g at 2.5%. Box with tube with 60 g at 5%. Box with tube with 60 g at 10%.
Storage recommendations:Store at room temperature to no more than 30 ° C. Keep the tube tightly closed
Legends of protection: Literature exclusively for physicians. Your purchase requires a medical prescription. Keep out of reach of children. Avoid contact with eyes, mucous membranes, hair and clothing, if accidentally occurs, wash with plenty of water.
Name and address of the laboratory: Manufactured in France by: Laboratoires Galderma, ZI Montdésir, 74540 Alby sur Chéran, France. Imported and distributed by: ALCON Laboratorios, SA de CV, Cda. of Popocatépetl No. 46, Col. General Anaya, Mexico, DF 03340.
Drug registration number: 023M94 SSA-IV.
IPPA code : BEAR-03361201697 / RM2004.




Reviews
There are no reviews yet.